CBD (Cannabidiol) for Cyclists: What We Know and What We Don't

Recently, CBD (Cannabidiol) has achieved near miracle status as being a cure all for just about everything, including; nausea, anxiety, cancer, arthritis, and even Alzheimer’s disease. As it has gained popularity it has found its way into cycling circles as a cure all, capable of relieving pain, shortening recovery times and enhancing workouts. So, what exactly is CBD and does it really work?
How is cannabidiol different from marijuana?
CBD is the abbreviation for cannabidiol, the second most prevalent of the active ingredients of cannabis (marijuana). While CBD is a component of marijuana it does not cause a “high.” According to a report from the World Health Organization, “In humans, CBD exhibits no effects indicative of any abuse or dependence potential…. To date, there is no evidence of public health related problems associated with the use of pure CBD.”
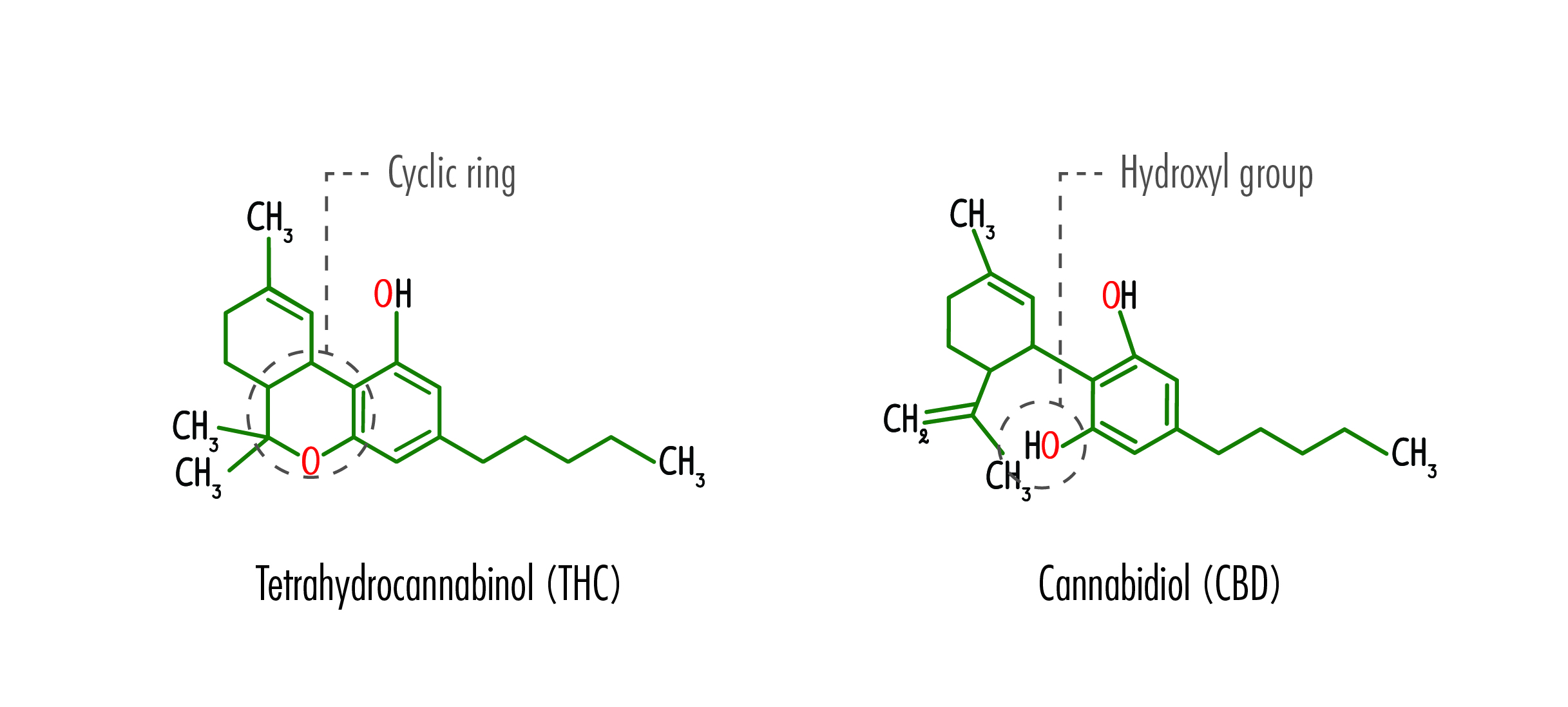
While both CBD and its sister cannabinoid THC have the same chemical formula (21 carbon, 30 hydrogen, and two oxygen) their structures differ significantly. So, while THC can bond with receptors in the brain, causing the high associated with marijuana, CBD cannot. Studies have shown that even high doses of oral CBD do not cause “THC-like effects” such as impairment, increased heart rate, or dry mouth.
Is CBD (Cannabidiol) Legal?
The answer is, well, in flux.
CBD is technically illegal under the federal 1970 Controlled Substances Act. As a Schedule I drug, it’s in the same category as drugs like heroin and LSD. That includes CBD derived from hemp, which unlike marijuana, contains only trace amounts of THC.

The DEA announced in September 2018 that cannabis-derived CBD products could have Schedule 5 status, as long as they have:
- THC levels of 0.1% or lower.
- Been approved by the U.S. Food and Drug Administration (FDA).
Several states have already legalized both marijuana and hemp, but users can technically be prosecuted under federal law. The passage of the 2018 Farm Bill furthered confusion about CBD legality:
The passage of the 2018 Farm Bill has led to the misperception that all products made from or containing hemp, including those made with CBD, are now legal to sell in interstate commerce. The result has been that storefronts and online retailers have flooded the market with these products, many with unsubstantiated therapeutic claims. FDA has seen CBD appear in a wide variety of products including foods, dietary supplements, veterinary products, and cosmetics. As this new market emerges, we have seen substantial interest from industry, consumers, and Congress. However, in the midst of the excitement and innovation, FDA’s role remains the same: to protect and promote the public health.
At present, any CBD food or purported dietary supplement products in interstate commerce is in violation of the FD&C Act due to the statutory provisions discussed above. However, FDA’s biggest concern is the marketing of CBD products that make unsubstantiated therapeutic claims to prevent, diagnose, mitigate, treat, or cure serious diseases, but have not obtained new drug approvals. For example, FDA has seen various CBD products with claims of curing cancer or treating Alzheimer’s disease. The proliferation of such products may deter consumers from seeking proven, safe medical therapies for serious illnesses – potentially endangering their health or life. FDA’s commitment to protect consumers from these unsubstantiated therapeutic claims does not just apply to CBD products – it is a longstanding commitment of the Agency across all the products we regulate.

CBD that comes from the marijuana plant is still illegal federally, since marijuana still has Schedule 1 drug status. That said, even the TSA recently announced it would allow people to fly with hemp products, including CBD produced within the regulations defined by the 2018 Farm Bill. While we wait for federal-level guidance, you should check your own state’s laws. That said, any kind of enforcement as it relates to CBD has been targeted toward the manufacturers and distributors — not the customers.
The Evidence for Cannabidiol Health Benefits
CBD has been touted as cure for a wide variety of health issues, but the strongest scientific evidence is for its effectiveness in treating some childhood epilepsy syndromes, such as Dravet syndrome and Lennox-Gastaut syndrome (LGS). These conditions typically don’t respond to antiseizure medications and for many parents and their children suffering from these cruel syndromes CBD truly has been a miracle drug. In numerous studies, CBD has reduced the number of seizures, and in some cases it has stopped them altogether. Some children have gone from hundreds of seizures a day to a handful or none at all. Recently the FDA approved the first ever cannabis-derived medicine for these conditions, Epidiolex, which contains CBD.

CBD is commonly used by people who suffer from anxiety and insomnia and studies suggest that CBD may help with both falling asleep and staying asleep.
CBD may also offer an option for treating different types of chronic pain. A study from the European Journal of Pain showed (in an animal model) that CBD applied on the skin could help lower pain and inflammation due to arthritis. Another study demonstrated the mechanism by which CBD inhibits inflammatory and neuropathic pain. While these results are promising, more study in humans is needed in this area to substantiate the claims of CBD proponents about pain control.
Is CBD (Cannabidiol) Safe?
Side effects of CBD include nausea, fatigue and irritability. The FDA strongly advises against using CBD if you are pregnant or breastfeeding. The FDA also notes that CBD can cause liver injury and interact negatively with other medications, you should speak with your doctor and/or pharmacist before trying a CBD product. Notably, CBD can increase the level in your blood of the blood thinner coumadin.
The most significant safety concern with CBD is that it is primarily marketed and sold as a supplement, not a medication. Currently, the FDA does not regulate the safety and purity of dietary supplements. Not only can you not know for sure that the product you buy has active ingredients at the dose listed on the label, but the product may contain other unknown and unlisted ingredients which may potentially be harmful.

A 2017 study published in The Journal of the American Medical Association found that 69 percent of the CBD products tested did not contain the amount of cannabidiol indicated on the label. Additionally, numerous CBD products have tested positive for heavy metals, like lead and mercury, as well as pesticides and bacterial and viral contamination.
Dangers with vaping products have been especially prominent recently. The Centers for Disease Control and Prevention (CDC) recommends that people not use THC-containing e-cigarette or vaping products, particularly those from informal sources like “friends, or family, or in-person or online dealers.” They have also added that “the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping products.”
Dosing CBD (Cannabidiol)
This is another area lacking research. There aren’t any hard and fast rules related to CBD dosing, because there have been no studies to define them.
There are a host of variables that come into play when dosing drugs; the condition being treated, the patient’s body mass, metabolic factors, age, how the drug is supplied (oral, topical, etc..) and so on. If you search online you’ll find resources like the CBD Dosage Calculator, but there is little to no research out there to support the guidance it provides.

CBD has very low oral bioavailability. So if you swallow CBD in pill form, only a fraction of that CBD will end up making it into the bloodstream and exert an effect in the brain or elsewhere in the body. Different routes of administration, such as vaporization, sublingual tinctures, or transdermal patches, provide a more direct route for CBD to enter the bloodstream. This probably allows for a larger proportion of the CBD in those products to enter the bloodstream. So, people interested in CBD products may want to consider experimenting with different routes of administration. A given amount of CBD in pill form could may not lead to the same outcomes as an identical amount taken via another route.
That said, the only real option when it comes to dosing CBD is to start with a low dose and slowly increase the dosage (titration) until you reach the relief you’re looking for.
Can You Overdose on CBD?
No fatal overdose cases with CBD have ever been reported. According to a study from 2011, chronic use and high doses up to 1,500 mg of CBD per day are well tolerated in humans.

Moreover, the Department of Health and Human Services reports there are “no signs of toxicity or serious side effects observed following chronic administration of cannabidiol to healthy volunteers, even in large acute doses of 700 mg per day.”
CBD Buying Tips
- ONLY PURCHASE PRODUCTS THAT HAVE BEEN INDEPENDENTLY TESTED (Look for ISO/IEC 17025 accredited products)
- CHECK THE PRODUCT’S COA (CERTIFICATE OF ANALYSIS). If that information is not available, choose a different product. A COA will include data on CBD concentrations and information on heavy metals found.
CBD for Cyclists
There is a growing body of research that supports the use of CBD as a treatment option for a range of health conditions, including inflammation and pain. However, there are also a lot of CBD products on the market making radical, unsubstantiated, snake-oil claims. As consumers, we must be well informed and diligent until the law and regulation catch up to the growing market. We also need to push for more and better research so we can better understand the potential of CBD, how to properly dose it and potential side effects.
Anecdotally, I’ve tested and am currently testing CBD products in various forms and will be bringing reviews to the site shortly. I wanted to cover the facts, as we know them, first.